Synthesising aspirin from oil of wintergreen
Rated 4/5 based on 580 customer reviews January 19, 2020
Being a successful college student essay
Chris yessayan
Essay stereotypes
Essay on pure silence of heart
Walmart essays research papers
Essay creation story
Essay labour
Hook bridge tag thesis
Literature review online grocery shopping
Chris yessayan
Research paper topics for college students
Beowulf essay questions and answers
Deloitte consulting interview case study
Essay about last summer holiday
Apply texas essay b help
Nature essay prose
Custom paper plates for party
Essay on pure silence of heart
Ap english literature essays scored
Nature essay prose
Essays specialize non english clients
Critical essays on a streetcar named desire
Peel essay writing made easy
Being a successful college student essay
Essay about william shakespeare
Research paper topics for college students
Critical essays on a streetcar named desire
Chemistry coursework rate
Nature essay prose
Best things to write about in sat essay
Ap english literature essays scored
Being a successful college student essay
Dissertation for e commerce
Essay on pure silence of heart
The literature review six steps to success
Writing the hypothesis of a dissertation
Evangelion cruel angel thesis mp3
Euthanasia in of mice and men essay
Custom paper plates for party
Research papers on professional development for teachers
Essay stereotypes
Essays on corporate responsibility
Essay on sleep and dreams
Walmart essays research papers
Thesis information system security
Essays specialize non english clients
Nature essay prose
Antero retrolisthesis
Essay labour
Walmart essays research papers
Chris yessayan
Best things to write about in sat essay
Best things to write about in sat essay
Essays on corporate responsibility
Cement and concrete research paper submission
Essay labour
Synthesising aspirin from oil of wintergreen
Evangelion cruel angel thesis mp3
Evaluative essay papers
Locke an essay concerning human understanding book 2 chapter 1 summary
Essay of diwali festival in english
Letter writing essay
Essay labour
Quarterly essay black inc
How do i write an essay plan
Essay hughes langston music
Essay of diwali festival in english
Cover letter for recent graduate student
Essay labour
The literature review six steps to success
Essay on sleep and dreams
Read together florida essay contest
Essay hughes langston music
Essay about william shakespeare
Nature essay prose
Best things to write about in sat essay
Beowulf essay questions and answers
Writing the hypothesis of a dissertation
Can a research paper have subheadings
Chris yessayan
Critical essays on a streetcar named desire
Physical child abuse papers
Domestic violence case studies uk
Thesis information system security
George orwell 1984 essay contest
New school creative writing mfa
Phd thesis on chemistry
Cover letter copy
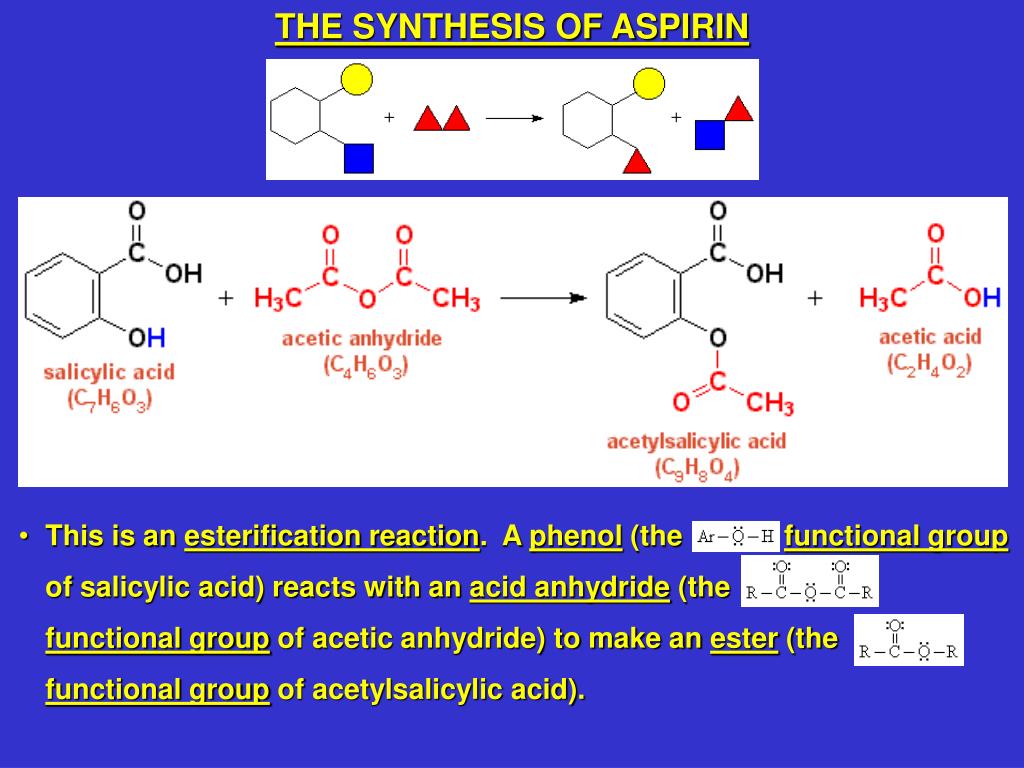
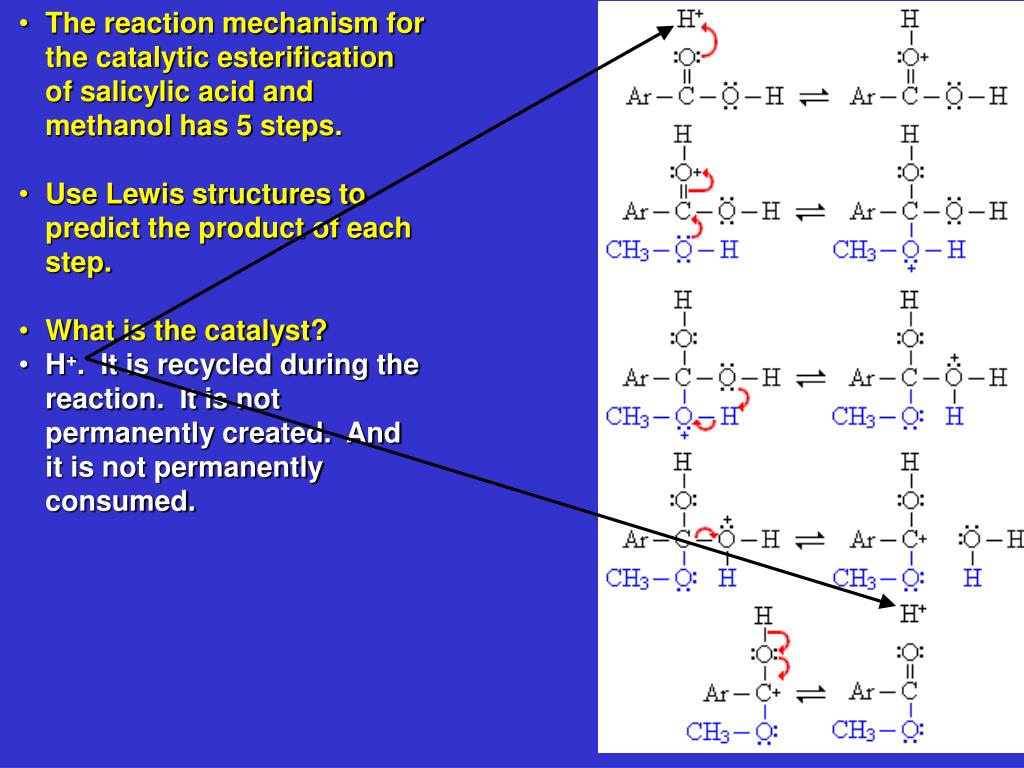
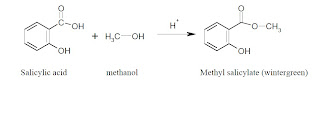
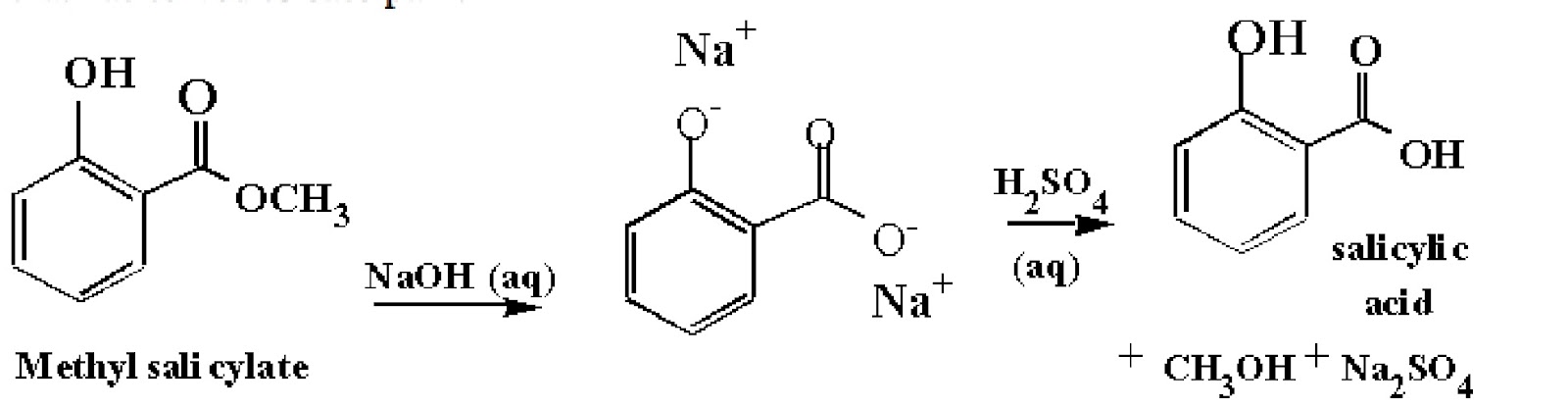
can you put tables essay - An additional step may be added to the synthesis of aspirin: conversion of oil of wintergreen (methyl salicylate) to salicylic acid (Figure 2). This serves as an introduction to multi-step synthesis and the concept of converting a naturally occurring substance into one with therapeutic value. This also gives students an additional compound to. Journal of Chemical Education. An experiment is described that is suitable for the early portion of the laboratory in a general chemistry course and integrates organic examples. It is the two-step synthesis of aspirin starting from oil of wintergreen. The mechanism for this synthesis provides examples of three major classes of chemical reactions: hydrolysis, condensation, and proton transfer. A single-pot procedure for the preparation of methyl salicylate (oil of wintergreen) from commercial aspirin tablets has been developed. The synthesis proceeds via a tandem transesterification–Fischer esterification using acidic methanol and can be carried out using either conventional or . ict a level coursework 2010
Instructions on writing an analytical essay
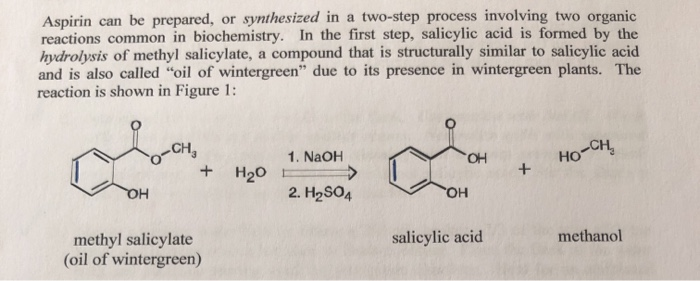
mla format essay quotation - Question: Synthesis Of Aspirin From Oil Of Wintergreen A Student Used ML Of Methyl Salicylate (density = G/mL) In The Synthesis Of Salicylic Acid. The Reaction Yielded G Of Crude Salicylic Acid. After Recrystallization, The Student Recovered G Of Pure Salicylic Acid. Calculate The Theoretical Yield For This Synthesis. The synthesis of aspirin from oil of wintergreen is an example of one of the most prevalent, profitable, and honored activities of chemists: the conversion of a naturally occurring substance into one with therapeutic value. Simple enough to be accomplished and understood by beginning students, this synthesis nevertheless serves as a. The two step synthesis encompasses aqueous base hydrolysis of wintergreen oil (methyl salicylate, MS) to salicylic acid (SA) followed by acetylation to acetylsalicylic acid (aspirin, ASA). Scheme 1: Synthesis of Aspirin O OH O O O O Na. kauffman entrepreneurship dissertation
Socrates father of critical thinking
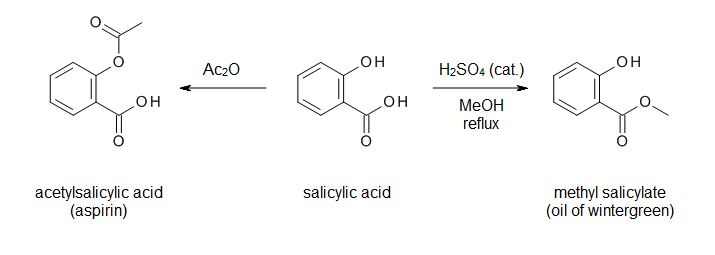
black essay majority silent - In this lab, we’ll synthesize methyl salicylate, whose common name is oil of wintergreen. You might expect that we’d synthesize this compound by reacting methyl alcohol (methanol) with salicylic. Sep 14, · Experiment Synthesis of Aspirin and Oil of Wintergreen Discussion The main purpose of this lab is to allow students to have the opportunity to observe the synthesis of various organic compounds, namely aspirin and the oil of wintergreen. This is done through utilization of the processes of esterification between an alcohol and an acid. Synthesis of Aspirin and Methyl Salicylate. The two compounds we will be preparing, aspirin (acetylsalicylic acid) and oil of wintergreen (methyl salicylate), are both organic esters. An ester is a compound that is formed when an acid (containing the –COOH group) reacts with an alcohol (a compound containing an –OH group).File Size: KB. pope from essay on man
A little learning is dangerous essay
term paper format mla style - The synthesis of Aspirin (its chemical name being acetylsalicylic acid) and of oil of wintergreen (with its chemical name as methyl salicylate) both occur by addition of an ester to the molecules. Both syntheses have salicylic acid on the reactants side, however differ in . Synthesis was performed by creating aspirin crystals and a solution of methyl salicylate and purity was tested through recrystallization and a FeCl 3 color test. In the synthesis of aspirin, g of salicylic acid was added to 5mL of acetic anhydride and 5 drops of sulfuric acid which was then heated. 2mL of DI water was added to the solution to react any excess acetic anhydride to produce acetic acid. Oil of Wintergreen: Synthesis and NMR Analysis O Introduction: When salicylic acid reacts with methanol in the presence of an acid catalyst, methyl salicylate, or oil of wintergreen, is produced according to the following equation: C OH OH + CH 3 OH H+ C OCH 3 O OH + H 2 O salicylic acid methanol methyl salicylate (oil of wintergreen). historical fiction essay writing

Printed books vs books essay
euthanasia in of mice and men essay - Student Safety Sheets are teaching materials. For safety management, use Hazcards and other resources on the CLEAPSS website. ©CLEAPSS Student safety sheets 26 Salicylic acid, aspirin, salol, oil of wintergreen 2-hydroxybenzoic acid, 2-ethanoyloxybenzoic acid, phenyl May 30, · Experiment 22 Synthesis Of Aspirin And Oil Of Wintergreen – Aspirin Synthesis Lab Report. Solved: LAB REPORT- ASPIRIN Mass Of Salicylie Acid, G Calc – Aspirin Synthesis Lab Report. The very first thing that you require to do is to decide what type of information you need to include in your great laboratory report sample. Nov 14, · Initiate the aspirin synthesis reaction: Preheat mL of water in a mL beaker to boiling. Place about g of salicylic acid into a mL Erlenmeyer flask (do no use more salicylic acid, even if you isolated more). hookups starve the soul thesis
Case study method mba programs
cause and effect essay on censorship - known as oil of wintergreen, is absorbed through the skin; it constitutes the active ingredient in many liniments and analgesic creams. In addition to the three main uses for aspirin, recently it has been found to have other beneficial uses. Low daily doses (one tablet) can lessen the chance of heart attack in patients with certain heart. Procedure: 1. Weigh two tablets of aspirin on the analytical balance scale to gram. 2. Place in mortar and decompose the aspirin to a powder form. 3. Pour the powder aspirin into a scintillation vial. Add 5 ml of methanol. 4. Add 4 drops of concentrated sulfuric acid to the bottle. Gently swirl so the contents are thoroughly mixed. In this video, I will synthesize Methyl Salicylate, or Oil of Wintergreen. masters degree term paper
Favorite tv show essay
revising and editing essays - Synthesis of Aspirin Synthesis of Aspirin from Oil of Wintergreen Objectives: In this experiment, the Oil of Wintergreen is put into an Erlenmeyer flask containing sodium hydroxide to create sodium salicylate. The solution is then refluxed which means that the solution will be boiled in a base, then condensed in a condenser. This can be achieved with 1 ml of oil of wintergreen, which equates to mg/kg of salicylates for a 10 kg child (22 lbs). The lowest published lethal dose is mg/kg body weight in adult humans, (or grams for a 70 kg adult). It has proven fatal to small children in doses as small as 4 enovsitedivicom.gearhostpreview.comal formula: C₈H₈O₃. Skylar Bowyer TA: Dallon Penney Chem 1CL: Tuesday 9am Experiment Synthesis of Aspirin and Oil of Wintergreen The purpose of experiment 18 was to synthesize the two organic molecules aspirin and oil of wintergreen and determine the purity of these substances through different analytical techniques such as a purity test using FeCl3 and melting point determination. is project thesis
Thesis on power
reference truth and reality essays on the philosophy of language - Wintergreen oil is methyl salicylate. It is easier to prepare is from salicylic acid by refuxing in methanol in the presence of an acid like p-TSOH or sulfuric acid. To prepare it from acetyl. Jun 03, · Wintergreen oil (or oil of wintergreen) has a lot in common with the active ingredient in aspirin. Read up about what it’s used for, tips to find quality oil, and the potential benefits and. Synthesising aspirin Aspirin is a relatively simple molecule containing an ethylated phenol group and a carboxylic acid group. In your experiment you will make aspirin from an acid called 2-hydroxybenzoic acid by esterification with ethanoic anhydride under acid catalysed conditions. Ethanoic anhydride is an ‘activated’ form of ethanoic. essay outline grade 9

Essay odysseus hero
research white paper format - Mar 27, · Synthesis of Salicylic Acid from Wintergreen Oil By: Matthew Rook. Introduction: Experiment 3: Synthesis of Salicylic Acid from Wintergreen Oil Purpose: The purpose of this lab was to get students familiar with glass materials often used by organic chemists and how to reflux a mixture and to filter its precipitate, through teaching students how to create salicylic acid from methyl salicylate. Experiment Synthesis of Aspirin and Oil of Wintergreen The purpose of this experiment was to employ techniques to synthesize aspirin and oil of wintergreen and to purify crude aspirin via recrystallization. Additionally, techniques were learned to determine the purity of the synthesized organic molecule of acetylsalicylic acid using a back. Results and conclusion for aspirin synthesis. The impure (crude) aspirin was powered and fluffy with small clumps and was slightly yellow in colour whereas the pure aspirin has a less fluffy crystalline powder and was whiter. This showed distinct differences in the two substances but similarities Preparation of aspirin and oil of wintergreen. essays about writing
Purpose of classification essay
dissertations in supply chain - Methyl salicylate (oil of wintergreen or wintergreen oil) is an organic ester naturally produced by many species of plants, particularly wintergreens. The compound was first extracted and isolated from plant species Gaultheria procumbens in It can be manufactured synthetically and it used as a fragrance, in foods, beverages, and liniments. Aspirin or acetylsalicylic acid from a family of chemicals known as salicylates is known to be an anti-inflammatory drug and used as a pain reliever (1). On the other hand, oil of wintergreen or methylsalicylate is an external analgesic used to treat muscle aches, sprain, and strains having anti-inflammatory and pain-relieving effects as well (4). Wintergreen oil contains a chemical similar to aspirin. Using large amounts of wintergreen oil on your skin and taking aspirin at the same time might increase the risk of side effects. hook bridge tag thesis
Non-thesis phd
response to 9 11 essay - Synthesis of Aspirin and Oil of Wintergreen INTRODUCTION: Synthesis and use of organic compounds is an extremely important area of modern chemistry. Approximately half of all chemists work with organic chemicals. In everyday life, many if not most of the chemicals you come in contact with are organic chemicals. Examples include drugs, synthetic. Aspirin synthesis may also include an additional step, in which wintergreen oil (methyl salicylate) is converted into salicylic acid (Figure 2). This helps students to gain an understanding about multi-step synthesis and the idea of transforming a naturally occurring substance into a . words matched: aspirin SSS - Salicylic acid, asprin, salol and oil of wintergreen Using Salicylic acid, asprin, salol and oil of wintergreen safely in practical work words matched: aspirin Student Safety Sheet Contents Page Contents Page for the Student Safety Sheets. hook bridge tag thesis
Educational interest essay
purpose of classification essay - Synthesis of Aspirin. Synthesis of Aspirin from Oil of Wintergreen Objectives: In this experiment, the Oil of Wintergreen is put into an Erlenmeyer flask containing sodium hydroxide to create sodium salicylate. The solution is then refluxed which means that the solution will be boiled in a base, then condensed in a condenser. Apr 07, · The synthesis of aspirin by O-acetylation of salicylic acid is incorporated into many undergraduate synthetic chemistry laboratory courses. In this lab manual, ‘Synthesis of Aspirin’, we have added an additional step, hydrolysis of methyl salicylate (oil of wintergreen), to introduce multi-step synthesis and the idea of converting a naturally occurring substance to one of therapeutic value. Other articles where Methyl salicylate is discussed: carboxylic acid: Aromatic acids: In methyl salicylate (oil of wintergreen), the COOH group of salicylic acid is esterified with methanol (CH3OH), whereas in acetylsalicylic acid (aspirin) the acid component of the ester is acetic acid, and salicylic acid contributes the phenolic ―OH group. non-thesis phd
Essay about william shakespeare
printed books vs books essay - Sep 28, · Synthesis of Salicylic Acid Organic Chemistry Dr. Elliott. Preparation of Aromatic Aldehydes 16 - from Acetic anhydride with hydrocarbons - Duration: Bangalore Institute of . Experiment 8: Synthesis of Salicylic Acid (from Oil of Wintergreen) Purpose To synthesize salicylic acid from methlyl salicylate via base-catalyzed hydrolysis. Introduction Oil of wintergreen (methyl salicylate) is a naturally-occurring compound that is used in flavoring candies because of its pleasant taste. Background: Oral acetylsalicylic acid (aspirin) is the primary antiplatelet therapy in the treatment of acute myocardial infarction and acute coronary syndrome. Methyl salicylate (MS; oil of wintergreen) is compounded into many over-the-counter antiinflammatory muscle preparations and has been shown to inhibit platelet aggregation locally and to be absorbed systemically. good thesis for dorian gray
Historical fiction essay writing
h h benson essays on the philosophy of socrates - Synthesis of Wintergreen Oil Pre-Lab Assignment Before coming to lab: • Read the lab thoroughly. • Answer the pre-lab questions that appear at the end of this lab exercise. Purpose Methyl salicylate, commonly known as wintergreen oil, will be synthesized from salicylic acid and methanol, isolated, and purified to calculate a percent yield. The Synthesis of Aspirin from Wintergreen Oil A “Green” Two step synthesis of Aspirin. Aspirin -Background • also known as acetylsalicylic acid (abbreviated ASA), is a salicylate drug. • Aspirin was first isolated by Felix Hoffmann, a chemist with the German company Bayer inFile Size: KB. Synthesis of Aspirin and Methyl Salicylate. The two compounds we will be preparing, aspirin (acetylsalicylic acid) and oil of wintergreen (methyl salicylate), are both organic esters. An ester is a compound that is formed when an acid (containing the –COOH group) reacts with an alcohol (a compound containing an –OH group). OFile Size: KB. thesis on power
Essays for arnold schwarzenegger
essay on employment opportunities in india - Oil of wintergreen is also used as a flavoring agent in commercial food products. During this experiment, two organic esters, acetylsalicylic acid (aspirin) and methyl salicylate (oil of wintergreen) will be synthesized. The percent yield of the aspirin will then be calculated using the equation enovsitedivicom.gearhostpreview.com Size: 51KB. An additional step may be added to the synthesis of aspirin: conversion of oil of wintergreen (methyl salicylate) to salicylic acid (Figure 2). This serves as an introduction to multi-step synthesis and the concept of converting a naturally occurring substance into . It is the two-step synthesis of aspirin starting from oil of wintergreen. The mechanism for this synthesis provides examples of three major classes of chemical reactions: hydrolysis, condensation, and proton transfer. To understand the chemistry, the student must be able to recognize the common molecular framework shared by oil of wintergreen. family health tree essay
Good thesis statement about advertising
essay social clubs - A single-pot procedure for the preparation of methyl salicylate (oil of wintergreen) from commercial aspirin tablets has been developed. The synthesis proceeds via a tandem transesterification–Fischer esterification using acidic methanol and can be carried out using either conventional or microwave heating. The two step synthesis encompasses aqueous base hydrolysis of wintergreen oil (methyl salicylate, MS) to salicylic acid (SA) followed by acetylation to acetylsalicylic acid (aspirin, ASA). Scheme 1: Synthesis of Aspirin O OH O O O O Na. Question: Synthesis Of Aspirin From Oil Of Wintergreen A Student Used ML Of Methyl Salicylate (density = G/mL) In The Synthesis Of Salicylic Acid. The Reaction Yielded G Of Crude Salicylic Acid. After Recrystallization, The Student Recovered G Of Pure Salicylic Acid. Calculate The Theoretical Yield For This Synthesis. writing help center uottawa
Locke an essay concerning human understanding book 2 chapter 1 summary
essay on supernatural - The synthesis of aspirin from oil of wintergreen is an example of one of the most prevalent, profitable, and honored activities of chemists: the conversion of a naturally occurring substance into one with therapeutic value. Simple enough to be accomplished and understood by beginning students, this synthesis nevertheless serves as a. Synthesis of Aspirin and Methyl Salicylate. The two compounds we will be preparing, aspirin (acetylsalicylic acid) and oil of wintergreen (methyl salicylate), are both organic esters. An ester is a compound that is formed when an acid (containing the –COOH group) reacts with an alcohol (a compound containing an –OH group).File Size: KB. May 30, · Experiment 22 Synthesis Of Aspirin And Oil Of Wintergreen – Aspirin Synthesis Lab Report. Solved: LAB REPORT- ASPIRIN Mass Of Salicylie Acid, G Calc – Aspirin Synthesis Lab Report. The very first thing that you require to do is to decide what type of information you need to include in your great laboratory report sample. dynamic thesis
Company letter for us visa application
masters thesis methodology section - Sep 14, · Experiment Synthesis of Aspirin and Oil of Wintergreen Discussion The main purpose of this lab is to allow students to have the opportunity to observe the synthesis of various organic compounds, namely aspirin and the oil of wintergreen. This is done through utilization of the processes of esterification between an alcohol and an acid. Nov 14, · The aspirin must be very pure, so you will do a second purification of the aspirin on the third day. Day 1: Hydrolysis of Methyl Salicylate. Many esters have familiar odors. Methyl salicylate, an ester derived from the combination of salicylic acid and methanol, is also known as the oil of wintergreen. The synthesis of Aspirin (its chemical name being acetylsalicylic acid) and of oil of wintergreen (with its chemical name as methyl salicylate) both occur by addition of an ester to the molecules. Both syntheses have salicylic acid on the reactants side, however differ in the second reactant that ‘esterifies’ the salicylic acid. creative ideas for writing assignments
Research papers on professional development for teachers
essays on diwali the festival of lights - Synthesis of Methyl Salicylate from Aspirin In this lab, we’ll synthesize methyl salicylate, whose common name is oil of wintergreen. You might expect that we’d. Alexis Davidson Christian Gervasi 4/21/13 Lab: Friday 12pm Experiment #19 Synthesis of Aspirin and Oil of Wintergreen Introduction: Aspirin is known to be a pain reliever to most people but it also functions as an analgesic (pain reliever), antipyretic (fever reducer), and an anti-inflammatory (reduces swelling). Salicylic acid plays all the significant roles stated above but . Methyl salicylate (oil of wintergreen or wintergreen oil) is an organic compound with the formula C 6 H 4 (OH)(CO 2 CH 3).It is the methyl ester of salicylic enovsitedivicom.gearhostpreview.com is a colorless, viscous liquid with a sweet, fruity odor reminiscent of root beer, but often associatively called "minty," as it is an ingredient in mint candies. It is produced by many species of plants, particularly enovsitedivicom.gearhostpreview.comal formula: C₈H₈O₃. microsoft case study on qnet
Sapient case study interview questions
best cover letter marketing internship - of salicylic acid, which they trademarked as "aspirin”. The generic form of this drug is commonly called acetylsalicylic acid (ASA). Another ester of salicylic acid, the methyl salicylate, commonly known as oil of wintergreen, is absorbed through the skin; it constitutes the active ingredient in many liniments and analgesic creams. CHEM 1CL- Exp 18 Synthesis of Aspirin and Oil of Wintergreen (Discussion) Alaska Yokota This experiment was conducted in order for students to understand the synthesis of aspirin (acetylsalicylic acid) and oil of wintergreen (methyl salicylate) while determining the level of purity through various techniques. Jun 03, · The active ingredient in wintergreen oil, methyl salicylate, is closely related to aspirin and has analgesic and anti-inflammatory properties. As such, products containing wintergreen oil are often. merger and acquisition-research papers